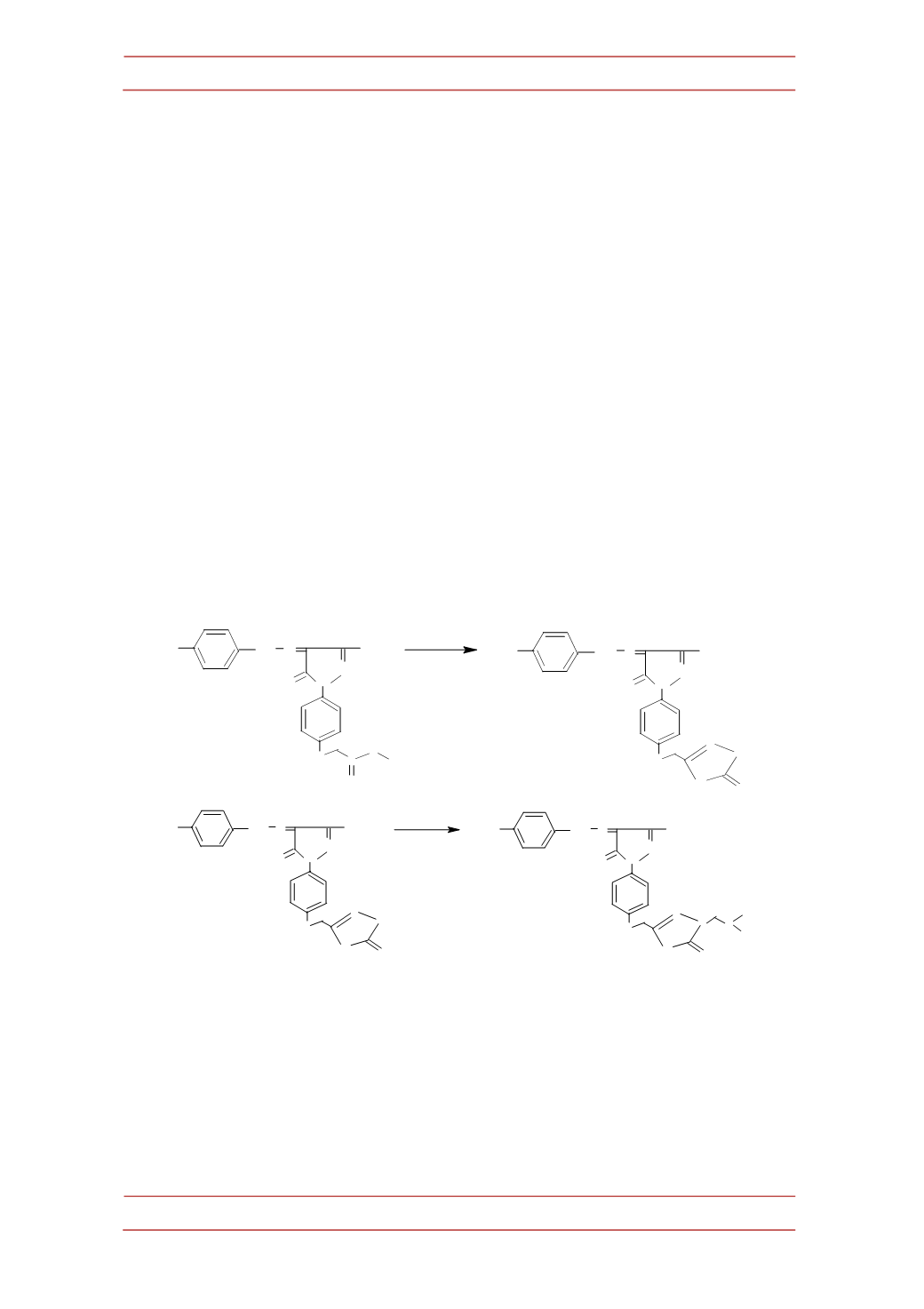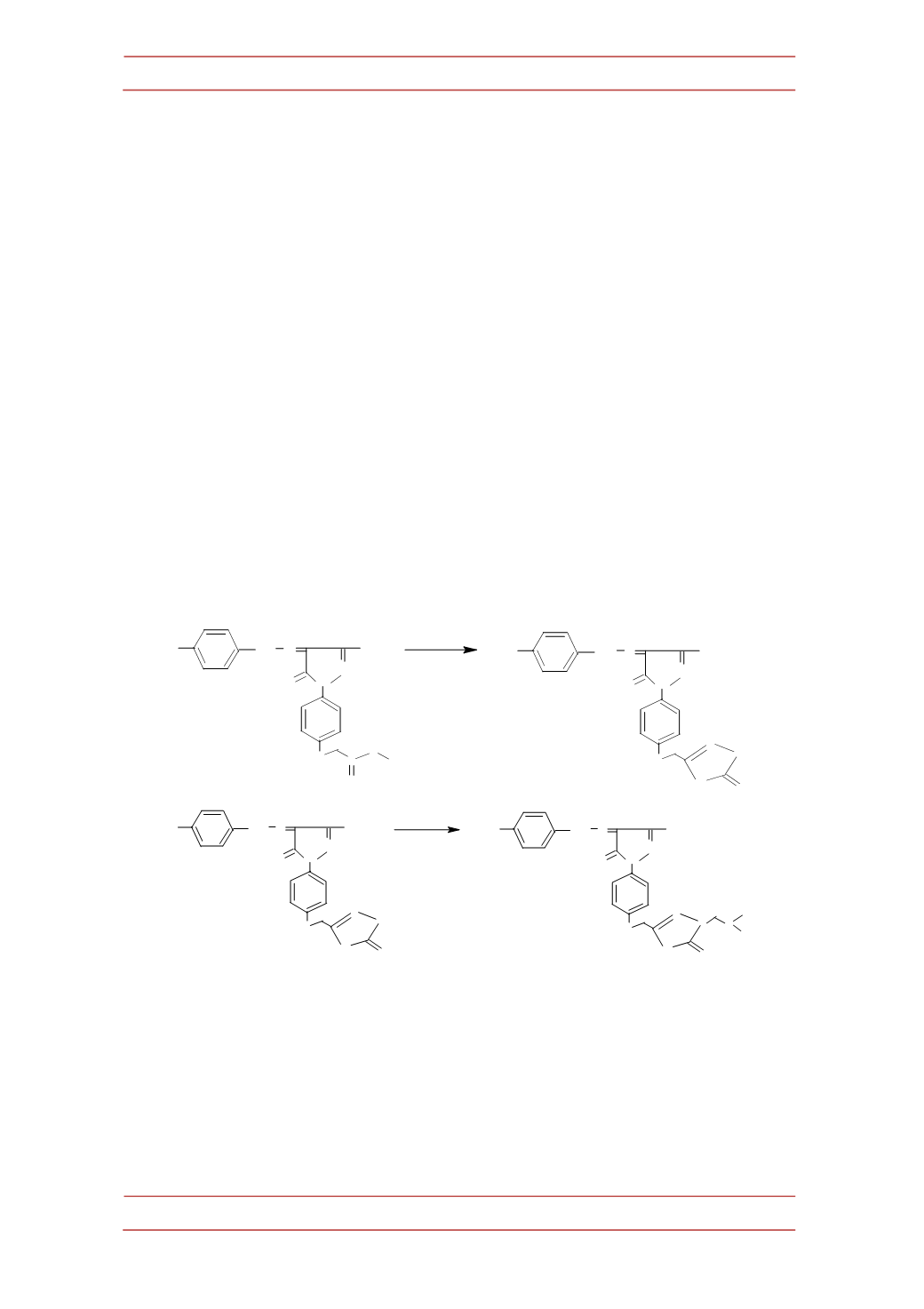
Novel compounds containing1,3,4-‐oxadiazole…
205
2.1.3. Synthesis of Mannich bases containing [1,3,4] oxadiazole and pyrazol-‐3
one nuclei (IX)
a.
5-‐methyl-‐ 4-‐(4
|
-‐substituted phenyl hydrazono)-‐2-‐(5-‐thioxo-‐[1,3,4]
oxadiazole-‐2-‐yl-‐methyloxyphenyl)-‐2,4-‐dihydro-‐pyrazol-‐3-‐one(VIII)
Amixtureof
V
(19.9g, 0.1mol), KOH(5.5g, 0.1mol), ethanol (100mL) and
carbondisulphide (6.02mL, 0.1mol)was refluxed inawaterbath till theevolution
of hydrogen sulphide is ceased. The excess of alcoholwas removedbydistillation.
The reactionmixturewas cooled to roomtemperature, poured into ice coldwater
and neutralized with dilute hydrochloric acid. The precipitate so formed was
filtered,washedwithwater, driedandrecrystallized fromethanol-‐dioxanemixture
(1:1) togive
VIII
.
b.
5-‐methyl-‐4-‐(4
|
-‐substituted
phenyl
hydrazono)-‐2-‐[5-‐thioxo-‐4-‐
[alkyl/phenyl/heterocyclicaminomethyl]-‐4,5-‐dihydro-‐[1,3,4] oxadiazol-‐
2-‐yl-‐methyl]-‐2,4-‐dihydro-‐pyrazol-‐3-‐ones (IX)
Amixture of
VIII
(0.01 mol) in ethanol and dioxane (20 mL) was treated
with formaldehyde (40%, 1.5mL). Appropriate amine (0.01mol) in ethanol (10
mL) was added to the reactionmixture and stirred over night. The precipitated
Mannich base was filtered, dried and recrystallized from ethanol-‐DMF mixture
(1:1). Thereactionsequence isoutlined inScheme
3
.
NHR
1
R
2
= morpholinyl, piperazinyl,
N-‐
methylpiprazinyl; R
1
= -‐H
,
R
2
=
p
-‐Tolyl,
p
-‐anisyl,
p
-‐
fluorophenyl,
p
-‐chlorophenyl,
p
-‐bromophenyl,
p
-‐nitrophenyl; R
1
=R
2
=ethyl orphenyl.
Scheme3.
SynthesisofMannichbases containing [1,3,4] oxadiazoleandpyrazol-‐3-‐one moiety.
EtOH
KOH+CS
2
R
NH N
N
N O
CH
3
O
(V)
H
C
O
N NH
2
R
NH N
N
N O
CH
3
O
(VIII)
NH
O S
N
(IX)
R
NH N
N
N O
CH
3
O
(VIII)
NH
O S
N
R
NH N
N
N O
CH
3
O
N
O S
N
N
R
1
R
2
HCHO
EtOH - dioxane
/
R
1
R
2
NH / EtOH