KrishnaNaik&col.
212
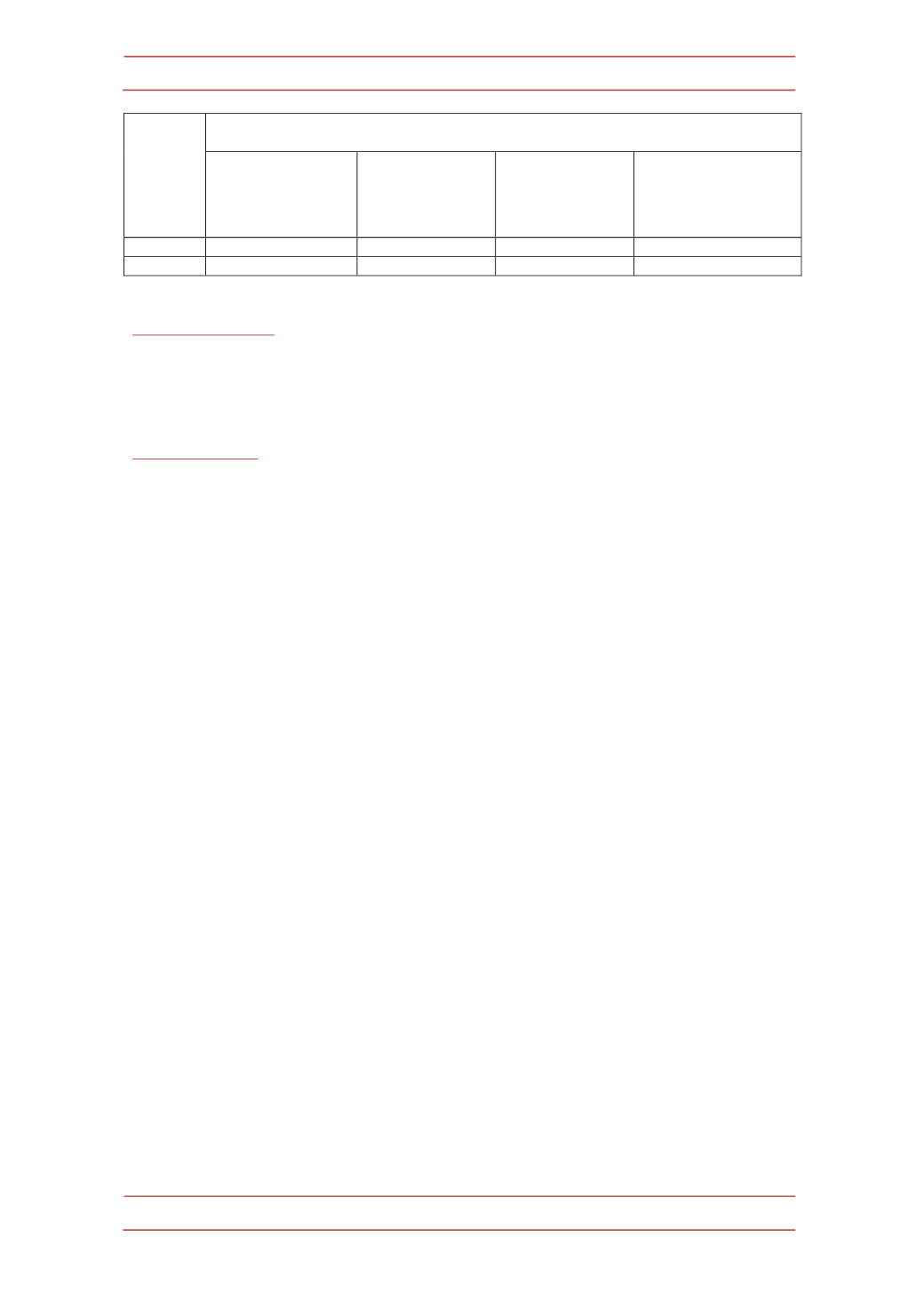
Compound
Zoneof inhibition inmm
(MIC inμg/mL)
Staphylococus
aureus
NCCS2079
BacillusCereus
NCCS2106
Escherichiacoli
NCCS2065
Pseudomanas
aeruginos
NCCS2200
IXj
2.75(32.5)
2.5(37.5)
2.25(40)
2.5(37.5)
IXk
2.5(35)
2.75(35)
2.25(37.5)
2.75(35)
4. CONCLUSSION
All the novel compounds have demonstrated moderate antimicrobial
activityagainst selectedof fungal andbacterial stains.
5. REFERENCES
1.
Ericsson, J.M.; Sherris, J.C.; Antibiotic sensitivity testing. Report of an International
CollaborativeStudy.
ActaPatholMicrobiol Scand.,
1971;217, 1-‐90
2.
Brown, B.A.; Wallace Jr., R.J.; Onyi, G.O. Activities of clarithromycin against eight slowly
growing species of nontuberculous mycobacteria, determined by using a broth
microdilutionMICsystem.
Antimicrob. AgentsChemother.,
1992
;
36, 1987-‐1990
3.
El-‐Kashef, H.S.; Abd-‐Alla, M.A.; Bayoumi, B E.; El-‐Timawy, A. A. M.; Synthesis and
antibacterial activity of some newpyrazolone dyes.
J. Chem. Technol. Biot.,
1983; 33, 294-‐
298 4.
Fan, X.; Zhang, X.; Zhou, L.; Keith, K.A.; Kernb, E. R.; Torrencea, P. F.; A pyrimidine-‐
pyrazolone nucleoside chimera with potent in vitro anti-‐orthopoxvirus activity.
Bioorg.
Med. Chem. Lett.,
2006;16, 3224-‐3228
5.
Guckian, K.; Carter, M.B.; Lin, E.Y.; Choi, M.; Sun, L.; Boriack-‐Sjodin, P. A.; Chuaqui, C.;
Lane B.; Cheung, K.; Ling, L.; Lee, W. C. Pyrazolone based TGFbetaR1 kinase inhibitors
.
Bioorg.Med. hem. Lett.,
2010; 20, 326-‐329
6.
Jignesh, P.R.; Arpita, B.S.; Nilesh, H.P.; Hemul, V.P.; Pradip, S.P.; Kashyap, K. B.; Kishor, R.D.
Synthesis and anti-‐tubercular activity of novel pyrazol-‐5(H)-‐one derivatives.
Eur. J. Chem.,
2011;2, 238-‐242
7.
Manojkumar, P.; Ravi, T.K.; Gopalakrishnan, S. Antioxidant and antibacterial studies of
arylazopyrazoles and arylhydrazonopyrazolones containing coumarinmoiety.
Eur. J. Med.
Chem.,
2009; 44, 4690-‐4694
8.
Chandrakantha, B.; Shetty, P.; Nambiyar, V.; Isloor, N.; Isloor, A.M. Synthesis,
characterization and biological activity of some new1,3,4-‐oxadiazole bearing 2-‐flouro-‐4-‐
methoxyphenylmoiety.
Eur. J.Med. Chem.,
2010; 45, 1206-‐1210
9.
Farshori, N.N.; Banday, M.R.; Ahmad, A.; Khan, AU.; Rauf, A. Synthesis, characterization,
and in vitro antimicrobial activities of 5-‐alkenyl/hydroxyalkenyl-‐2-‐phenylamine-‐1,3,4-‐
oxadiazoles and thiadiazoles.
Bioorg.Med. Chem.Lett.,
2010; 15, 1933-‐1938
10. Hussain, A.; Ajmal, M. Synthesis of novel 1,3,4-‐oxadiazole derivatives and their biological
properties.
ActaPharm.,
2009
;
59, 223-‐233
11. Rakesh, S.; Awani, K.R.; Kesari, A.N.; Yar, M.S. Synthesis and biological evaluation of 2,5-‐
disubstituted1,3,4-‐oxadiazole.
Asian J. Res. Chem
., 2009; 2, 34-‐42
12. Rakesh, C.; Anshu, A.;Manoj Kumar, P.; Chander Sharma, P.; Sukumar,M.; Thengungal Ravi,
K.Synthesis of novel 1,3,4-‐oxadiazole derivatives as potential antimicrobial agents.
Acta
Pol. P arm
., 2010; 67, 247-‐253


