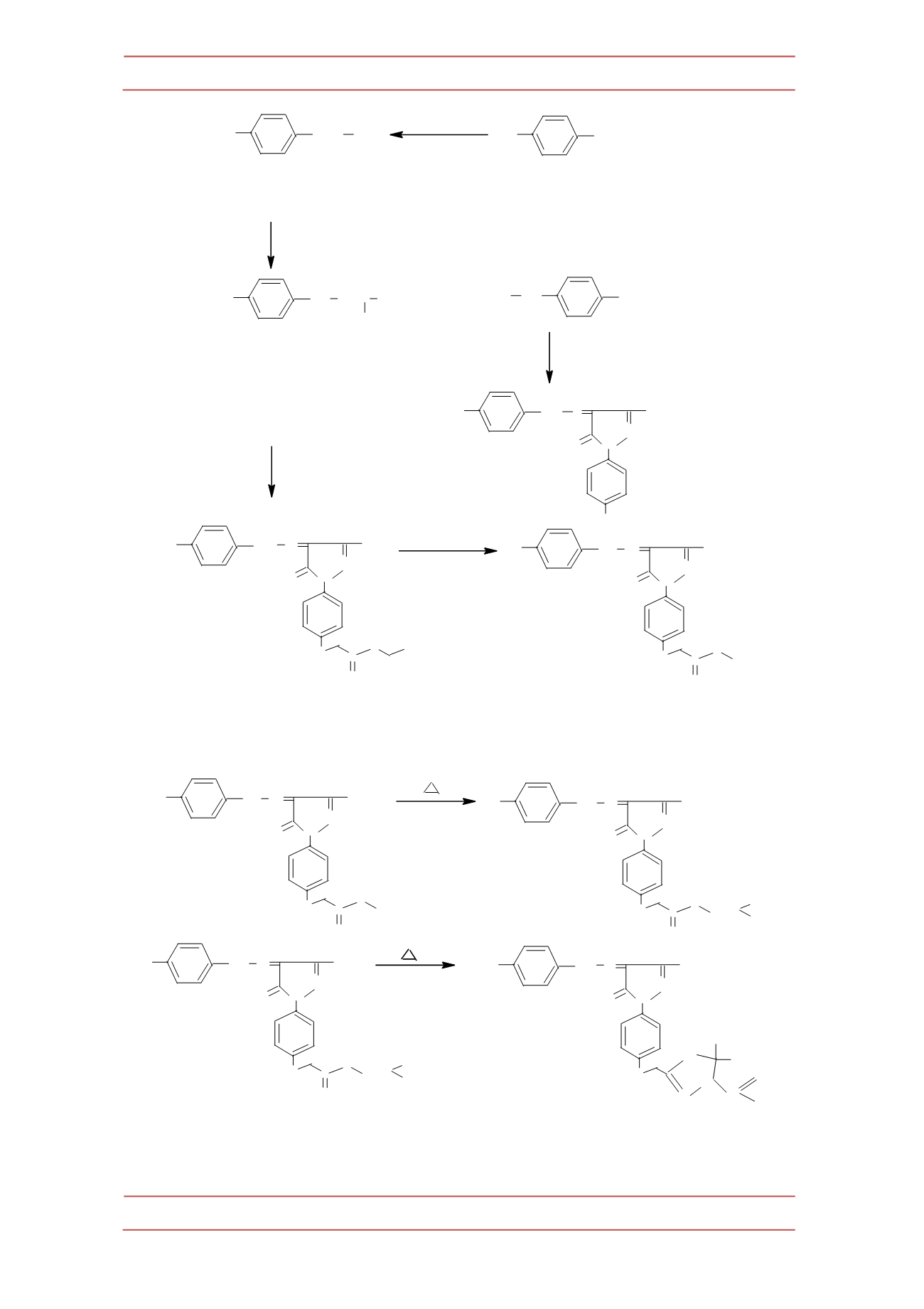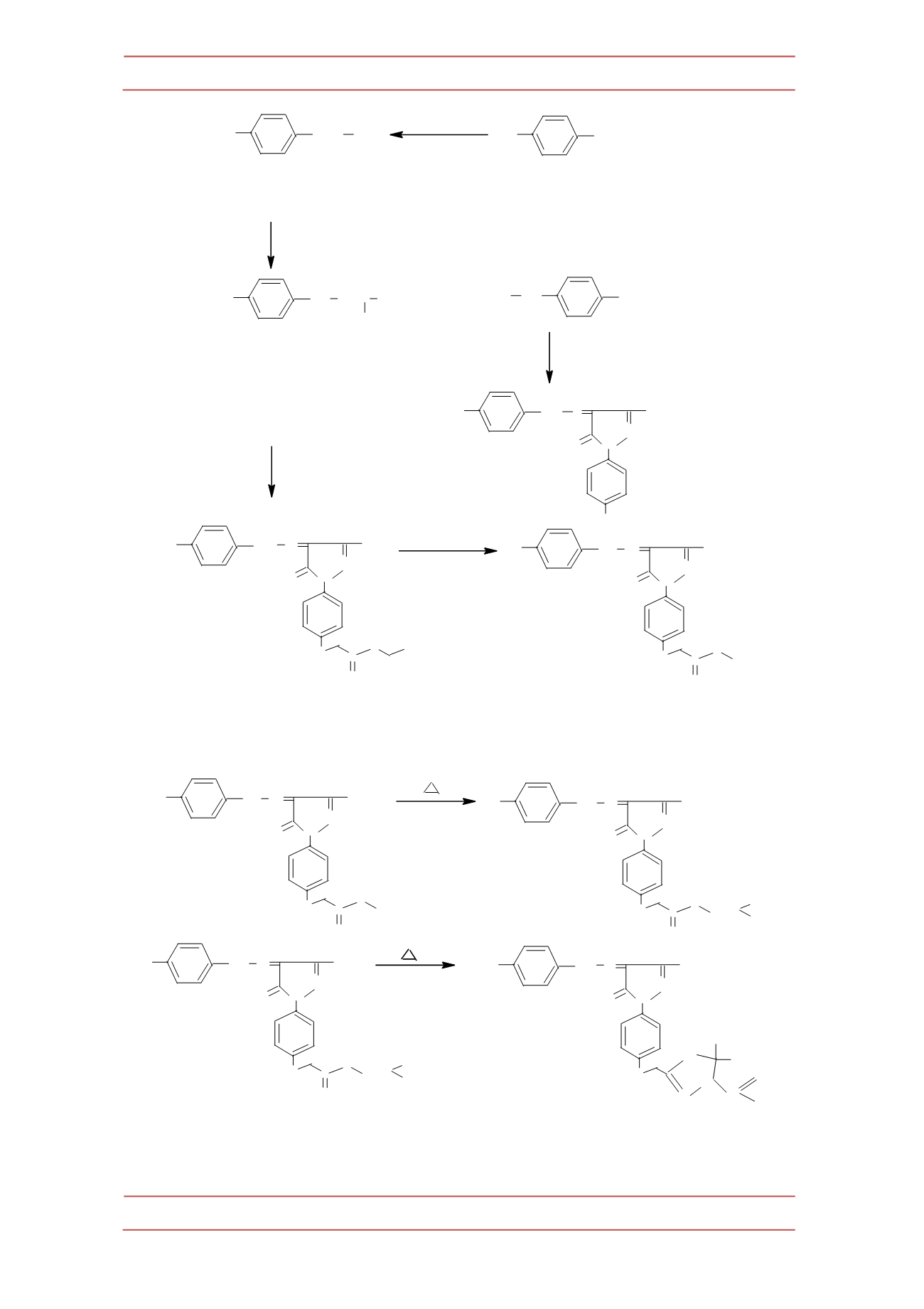
KrishnaNaik&col.
204
R=H, CH
3
, OCH
3
, OC
2
H
5
, Cl, Br.
Scheme 1.-‐
Synthesis of {4-‐[3-‐Methyl-‐5-‐oxo-‐4-‐(4
|
-‐substituted phenyl hydrazono)-‐4,5-‐dihydro-‐
pyrazol-‐1-‐yl]-‐phenoxy}-‐aceticacidhydrazide (
V
).
R = -H, -CH
3,
-OCH
3,
-OC
2
H
5,
-Cl, -Br, R
1
= -CH
3
, R
2
= -C
6
H
5,
p
-
CH
3
C
6
H
4,
p
-ClC
6
H
4,
p
-OCH
3
C
6
H
4,
p
-NO
2
C
6
H
4.
Scheme 2.-‐
Synthesis of 2-‐(4-‐acetyl-‐5-‐methyl-‐5-‐phenyl-‐4,5-‐dihydro-‐[1,3,4]oxadiazole-‐2-‐
yl(methyl)-‐5-‐methyl-‐4(4
|
-‐phenyl hydrazono)-‐2,4-‐dihydrazono-‐pyrazol-‐3-‐one (VII).
+
CH
3
COCH
2
COOC
2
H
5
CH
3
COONa
ClCH
2
COOC
2
H
5
+
DMF
K
2
O
3
(anhydrous)
R
NH
N
N O
CH
3
OH
N
+
R
NH
COOC
2
H
5
N=C COCH
3
NH
OH
H
2
N
R
NH
2
NaNO
2
+HCl
0 - 5
o
C
R
N=N Cl
(I)
(II)
(III)
N
2
H
4
, H
2
O
Ethanol
R
R
NH
N
N O
CH
3
N
(IV)
O C
O
O
NH N
N
N O
CH
3
O
(V)
H
C
O
N NH
2
DMF
Microwave
irradiation
150W
R
NH N
N
N O
CH
3
O
(V)
H
C
O
N NH
2
(VI)
R
NH N
N
N O
CH
3
O
H
C
O
N N=C
R
1
R
2
glacial AcOH
Acetophenone /
R
NH N
N
N O
CH
3
O
(VII)
CN
N
O R
2
R
1
O
CH
3
Aceticanhdride
(VI)
R
NH N
N
N O
CH
3
O
H
C
O
N N=C
R
1
R
2