Class I phosphoinositide3-‐kinases in immunity…
95
least in certain cases Ras-‐family proteinsmight play an active role in recruiting to
membranes catalyticclass IA(15) andclass IBsubunits (10).
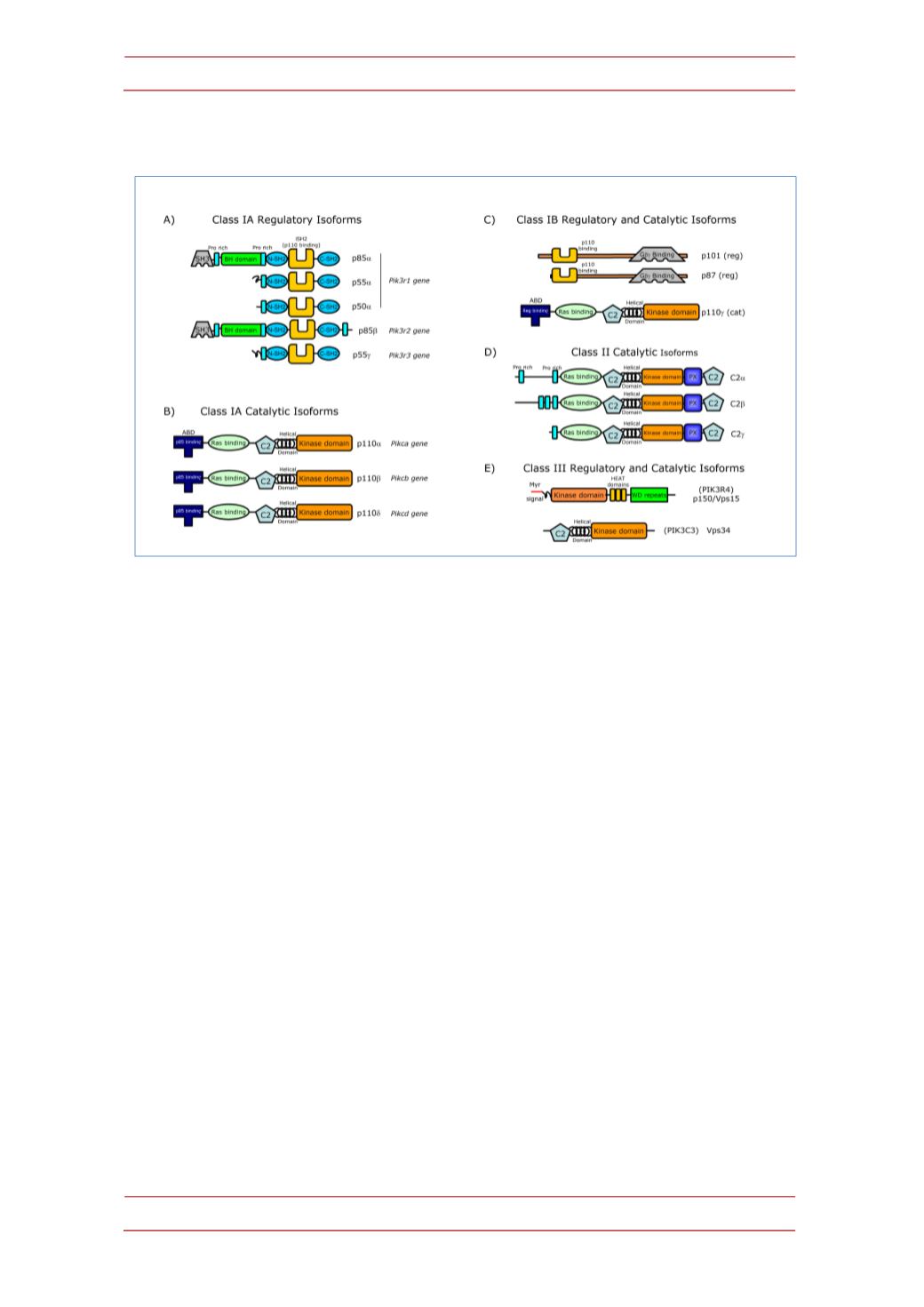
Figure 2.
Structure of the catalytic and regulatory subunits of mammalian PI3K classes and
subclasses. Size in kDa is indicated in the name of class I PI3K, all proteins are represented at the
same approximate scale. PI3K catalytic subunits contain a common core of one C2 domain, one
helical domain, and one catalytic kinase domain. Class IA and IB catalytic subunits (
α
,
β
,
δ
, and
γ
)
are the product of single genes ; they have two domainsN-‐terminal to the core, namely an adaptor
bindingdomain (ABD) that binds toregulatory subunit, andoneRas-‐bindingdomainwherebinding
of Ras family proteins activate the kinase activity.
A; B)
Class IA catalytic subunits (
α
,
β
, and
δ
)
associate with any class IA regulatory subunits encoded by three different genes.
Pik3r1
can
produce three different proteins (p85
α
, p55
α
and p50
α
) sharing one Pro-‐rich region, plus one N-‐
terminal andoneC-‐terminal SH2domains separatedbyand inter-‐SH2domain (iSH2) that binds the
ABD domain in the catalytic
α
,
β
, and
δ
subunits. The p85
α
subunit has one N-‐terminal SH3, one
Proline-‐rich, and one BH (BCR homology) domain; the p85
β
subunit coded by the
Pik3r2
gene is
similar top85
α
subunit but has anadditional c-‐terminal Pro-‐rich region. Thep55
γ
subunit encoded
by
Pik3r3
and p55
α
have similar structures.
C)
Class IB catalytic subunits (p110
γ
) bind to p87 or
p101 class IB regulatory subunits endowed with domains able of associating to the G
α
and G
β
subunits of heterotrimeric Guanine nucleotide-‐binding proteins (G proteins) that initiate signals
deliveredbyG-‐proteincoupledreceptors (GPCR).
D)
Class II PI3Ksdonot have regulatory subunits,
and seem to be constitutively bound to intracellularmembranes. They have a role in different cell
functions including cell migration, exocytosis, and apoptosis, but the precisemechanisms involved
are not clear. The Class III catalytic subunit Vps34 (Vacuolar protein sorting 34, also termed
PIK3C3) is part of a heterodimer with the myristoylated protein Vps15, that is located in the cell
membranes and form larger multi-‐protein complexes depending on the particular vesicle traffic
process considered (autophagy, phagocytosis, endosome traffic). Vps15 has a kinase domain
probably inactive, HEAT domains containing anti-‐parallel
α
-‐helices involved in protein-‐protein
interactions; andWDrepeats that serveas scaffolds for interactionwithotherproteins.
Regulatory subunits inhibit the catalytic activity of the p110 subunits but
also prevent their degradation. Activation of PI3K begins upon recruitment of the
enzyme complex through regulatory subunits to the inner side of membrane
bilayers where their substrate is located, and where interaction with the
negatively charged surface through positively charged aminoacid residues further
stabilizes its location. This is followed by interaction with and activation of the


