REVISIÓN |
The role of the catecholaminergic pathway in heart development: beyond neuromodulatory actions
Catalina Hernández-Sánchez, Patricia Vázquez, Flora de Pablo*
3D Lab (Development, Differentiation, Degeneration). Centro de Investigaciones Biológicas, Ramiro de Maeztu 9, Madrid 28040. Spain
*e-mail: fdepablo@cib.csic.es
An. Real Acad. Farm. Vol. 80, Nľ 2 (2014), pag. 347-361.
abstract
Hormones are expressed during development in unexpected locations and stages, and this fact relates to their distinct functional roles in the embryo. In recent work, we found that the expression of Tyrosine Hydroxylase (TH, first enzyme of the catecholamine synthetic pathway) and the presence of catecholamines, antecede neural innervation in some tissues. We focus this overview on the vertebrate developing heart. TH transcripts were present in early cardiogenesis, and adrenergic as well as dopaminergic receptors were found in the cardiac region of chick embryos. We found direct effects of dopamine on cardiac gene expression and we have advanced in revealing the function of catecholamines on cardiac patterning. |
Keywords: tyroxine hydroxylase; catecholamines; dopamine; cardiogenesis.
resumen
El papel de las catecolaminas en el desarrollo del corazón: más allá de sus acciones neuromoduladoras
Las hormonas están expresadas durante el desarrollo en etapas y localizaciones inesperadas y este hecho se relaciona con sus distintas funciones en el embrión. Recientemente, hemos encontrado que la expresión de la Tirosina Hidroxilasa (TH, el primer enzima de la ruta de síntesis de catecolaminas) y la presencia de catecolaminas, anteceden a la inervación neural en algunos tejidos. Este artículo está centrado en el desarrollo del corazón de vertebrados. Los transcritos de TH se expresan durante la cardiogénesis temprana y se encontraron receptores dopaminérgicos y adrenérgicos en la región cardiaca del embrión de pollo. Hemos demostrado efectos directos de la dopamina sobre la expresión de genes cardiacos y hemos avanzado en caracterizar una función de las catecolaminas sobre la formación del patrón del corazón. |
Palabras clave: tirosina hidroxilasa; catecolaminas; dopamina; cardiogénesis.
1. INTRODUCtioN
Hormones and neurotransmitters synthesis localized, under traditional views, within the endocrine and nervous systems, respectively. In contrast, others and we have found expression of hormones much earlier in development than the emergence of the endocrine gland especialized in their secretion takes place. For example, growth hormone, secreted in adult organisms by the pituitary gland, is detected in the blastocyst and many extrapituitary tissues during development (1).
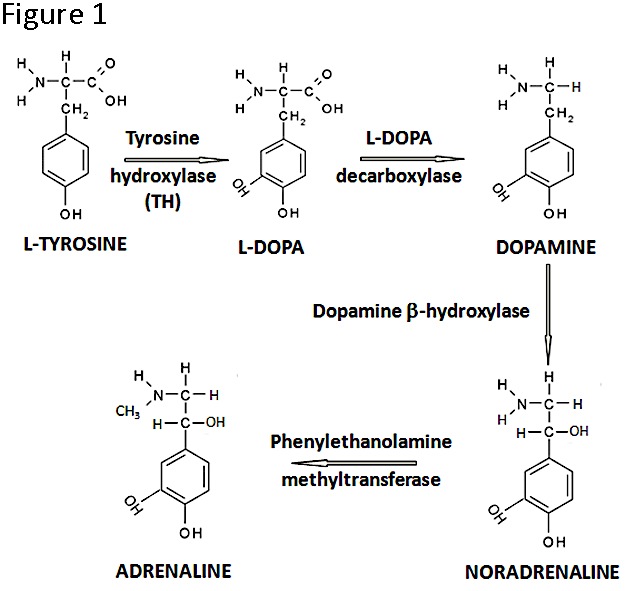
Figure 1. The catecholamine biosynthesis pathway. Note that TH is the rate-limiting enzyme and that in developing tissues the pathway may not lead to the production of all three catecholamines.
It appears that tissue GH may act in paracrine or autocrine fashion to control proliferation and differentiation, although a full characterization is not avalilable. In studies carried out over the last three decades, we showed that the expression of proinsulin (molecular precursor form of insulin) antecedes the development of the pancreas (2, 3) and it has a cell survival function in the developing nervous system (4, 5). Proinsulin expression is tightly regulated as well in the heart tube, and malformations are induced when proinsulin is disproportionally high at early stages (6).
Catecholamines are well known essential hormones/neurotransmitters during postnatal life, when they have cardiovascular, neuromuscular and behavioral effects, but their function in specific organs during vertebrate development is poorly characterized (7). The catecholamine synthetic pathway (Figure 1) is initiated by the action of Tyrosine Hydroxylase (TH) which catalyses the conversion of the amino acid L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA); in subsequent reactions, dopamine, noradrenaline and adrenaline may be produced. While we were studying proinsulin transcripts regulation in development we found, to our surprise, non-canonical th-insulin chimeric mRNA transcripts (8); this finding motivated further work on the presence and function of TH in early embryogenesis.
2. Tyroxine Hydroxylase, a gene with complex transcriptional regulation
As commented above, genes/proteins have mechanisms of regulation and functions at certain developmental stages of vertebrates distinct from their canonical roles later in life. In this context, while the th and ins genes are located in tandem in the same orientation (and they are generally transcribed independently), two chimeric transcripts containing exons from both of these genes can also be produced in a regulated manner during the first few days of development in the chick and quail embryos (Figure 2).
It is estimated that between 2 and 5% of tandem human genes may be transcribed into chimeric mRNAs (9-11), although the function of these unusual transcripts is in general unknown.
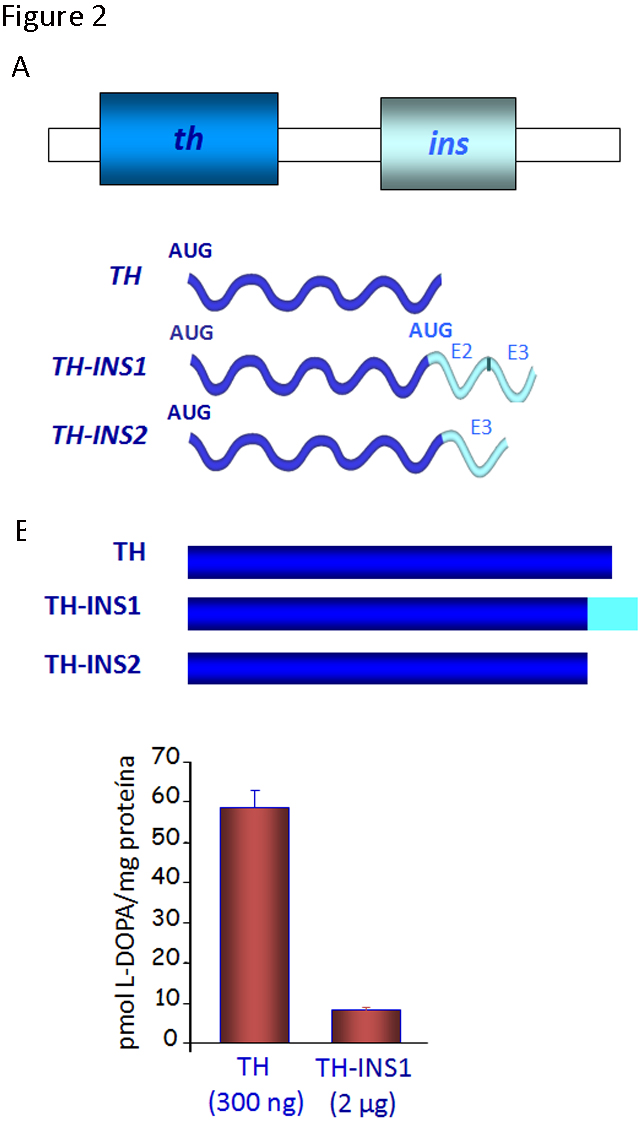
Figure 2. Novel forms of gene regulation in the th-ins locus. A) The schematic genomic organization of the chicken th-ins locus (there is a similar cluster in mammals although the transcriptional regulation may be different) and diagram of the transcripts generated by the out-of-frame splicing between th exon 13 and exon 2 or 3 of the insulin gene. B) TH isoforms generated and their enzymatic activity was analyzed by HPLC for L-DOPA production. The extracts of transfected HEK293T cells with these TH isoforms show a lower enzymatic activity than the canonical TH. (Modified from [8])
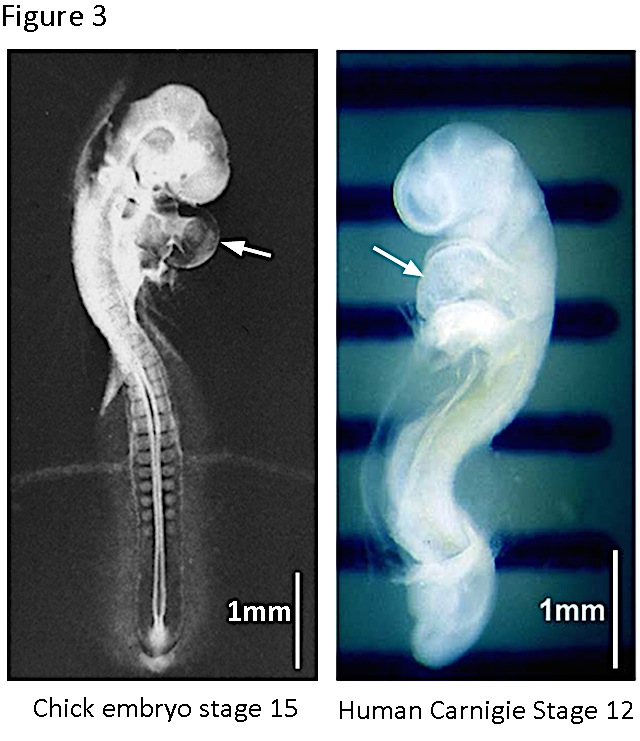
Figure 3. The chick and the human embryos at early stages of cardiogenesis. The chick embryo reaches the stage 15 ([16]) at ~2.5 days of incubation. The heart tube is already turned and specified in auricular and ventricular regions. The human embryo reaches the stage 12 (Carnigie classification, http://php.med.unsw.edu.au/embryology/index.php?title=Carnegie_Stages) at 26-30 days of pregnancy. The heart prominence is indicated by the arrow. Note the great similarity of both embryos at these stages.
The chimeric transcripts containing exons from th and ins genes give rise to two novel isoforms of the TH protein (TH-INS1 and 2) with markedly reduced functionality, when compared to the canonical TH. The levels of L-DOPA in cells expressing TH-INS1 are approximately 5-fold lower than those found in cells expressing similar or lower amounts of TH protein (Figure 2). In addition, the TH-INS1 chimeric mRNA also generates a small amount of insulin (8). Besides this unusual post-transcriptional regulation, the th gene displays an unexpected expression pattern.
We found that the expression of th mRNA in the chick embryo antecedes development of the nervous system. th transcripts were detected from gastrulation onwards, and they were enriched in the cardiogenic region. Before focusing this overview in the heart TH during cardiogenesis, it is worth however to emphasize the remarkably similar features of a human embryo and a chick embryo during that period (Figure 3). In vertebrates, the heart is initially formed by the fusion of the two bilateral endocardial tubes arising from the splanchnic lateral plate mesoderm. The resultant primitive heart tube, located at the ventral midline of the organism, undergoes a complex series of movements and tissue remodeling events that leads to the formation of the mature chambered organ (12).
3. Use of the chick embryo as a model for studying the role of TH in cardiogenesis
As very clearly stated by Brand (in a kind editorial comment of our work, 13), “despite its evolutionary distance to mammals, the chick embryo is a valuable model to work out the mechanism of cardiac specification. The embryo is large and accessible and, therefore, manipulations at the time of cardiac specification and early heart formation are easily performed”. Research in the last two decades utilizing this model organism has identified several signaling molecules that are important for cardiac induction (14).
The fact that the chick embryo can be accessed and manipulated without disrupting early development, allows to perform experiments of gain of function and loss of function starting during gastrulation, and to analyze the effects on embryonic organogenesis (Figure 4). Factors or antibodies may be added and, if the aim is to look for effects over the next several days, the embryo can be reached through a window in the shell and incubation can continue. To look for short-term effects, as in the series of studies reviewed here, the addition of molecules or plasmid DNA was carried out maintaining the embryo in culture under specific tension conditions (15). The chick embryos were treated at stages 3-5 (12-22 hours of development, according to Hamburger and Hamilton (16) and were studied at stages 11-12 (less than 50 hours of development). The addition of dopamine or enzymatic inhibitors of TH activity could be carried out using heparin or resin microbeads, implanted in the embryo underneath the epiblast (Figure 4B). The uptake by the embryo tissues of plasmid DNA containing TH, GFP (Green Fluorescence Protein) or TH antisense-morpholino DNA was facilitated by electroporation (Figure 4C).
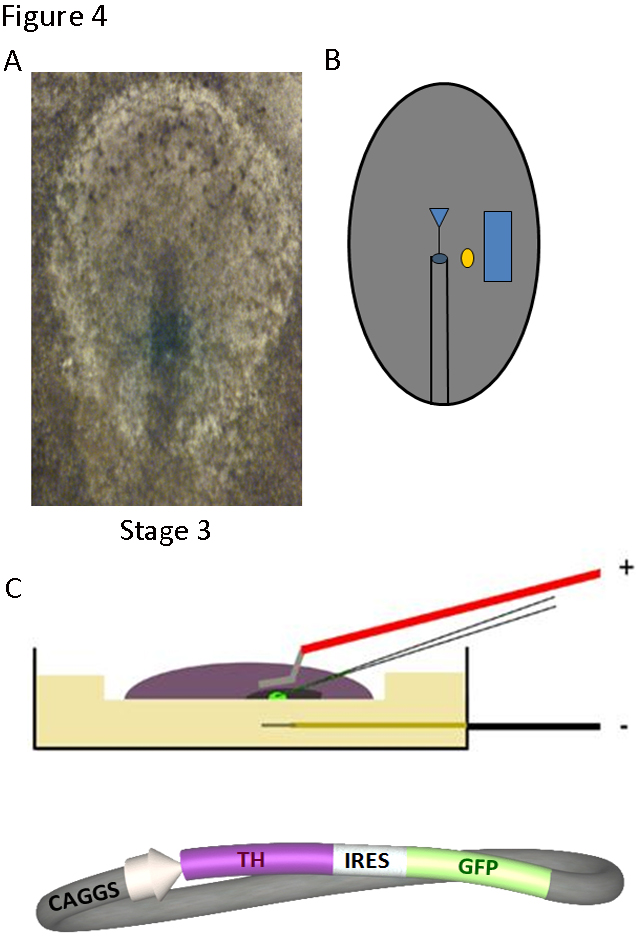
Figure 4. Techniques to manipulate gene expression in the chick embryo: implanted microbeads and electroporation. A) The stage 3-5 blastoderm can be accessed in ovo and B) a factor-coated microbead (yellow circle) can be implanted at the desired location lateral to the heart field of one side (blue square). After 6-18 h of further incubation the embryo can be retrieved and processed for RNA or protein detection. C) The blastoderm can also be placed in culture and injected and electroporated with DNA constructs, such as that shown in schematic form, in which the sequences of TH and the green fluorescence protein (GFP) are linked by an IRES (Internal Ribosome Entry Site). After 18-36 h of additional incubation, embryos can be collected and processed.
TH as well as adrenergic and dopaminergic receptors are expressed in the chick embryo developing heart.
We have found that th mRNA expression is enriched in the cardiac field of gastrulating chick embryos. By stages 8-10, it was restricted to the splanchnic mesoderm of both endocardial tubes and was subsequently (stages 11-12) expressed predominantly in the myocardial layer of the atrial segment (Figure 5, (13)). The levels of th mRNA in the heart increased several fold between stage 8 and stage 12 (Figure 5).
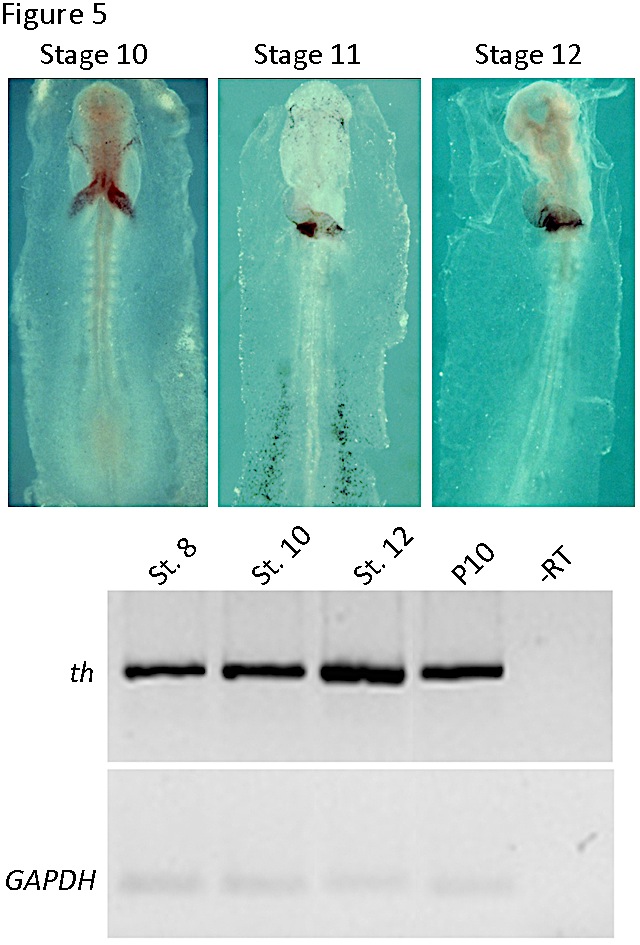
Figure 5. Tyrosine hydroxylase expression in the chick embryonic heart. In the upper panel, whole mount in situ hybridization for stages 10 to 12 are shown. Note the restricted expression in the heart tube. In the lower panel RT-PCR of th mRNA expression is shown in the developing endocardial tube (stage 8) and heart tube (stages 10 and 12) and postnatal day 10 (P10) heart. An internal control with GAPDH is shown below. (Modified from [13]).
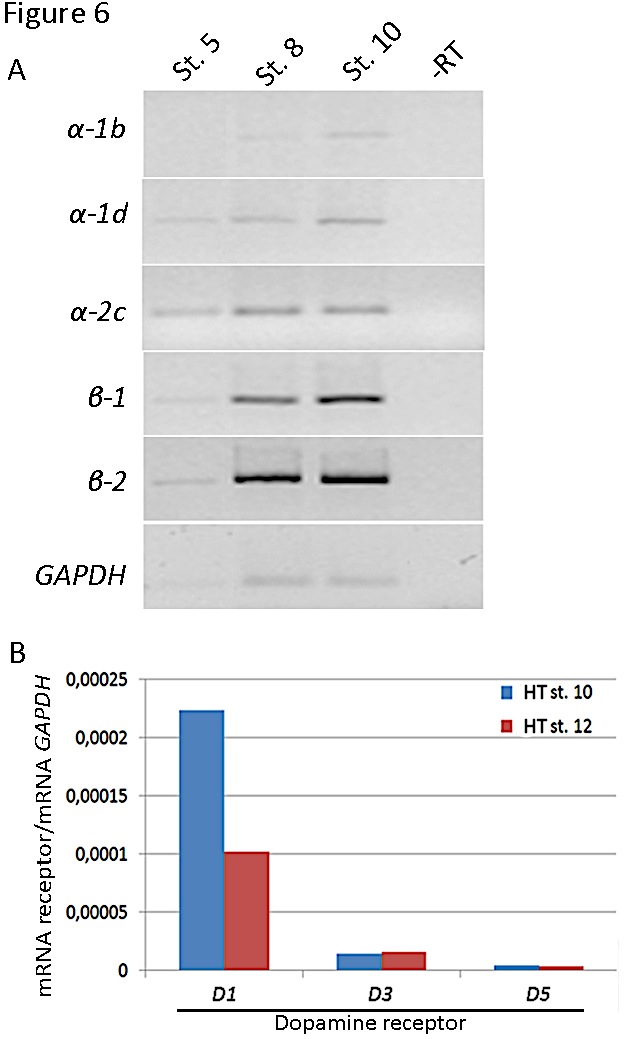
Figure 6. Expression of receptors in the chick embryonic heart regions. A) Analysis of adrenergic receptors (a-1b, a-1d and a-2c ; b-1 and b-2) expression by RT-PCR of mRNA from the precardiogenic area (stage 5), endocardial tubes (stage 8) or heart tube (stage 10). An internal control with GAPDH is shown below. B) Analysis of dopaminergic receptors (D1, D3 and D5) expression by quantitative PCR of mRNA from stage 10 and 12 heart tubes. The levels of dopamine receptor transcripts were normalized relative to GAPDH mRNA levels. Primers are shown in Tables 1 and 2.
We set out, then, to demonstrate the presence of receptors which may mediate the action of catecholamines, likely produced by the cardiomyocytes of the primitive heart tube. Adrenergic and dopaminergic receptors are 7-transmembrane domain G protein-coupled receptors. The family of adrenergic receptors are composed by α- and β-receptors. There are two types of α-receptors: The stimulation of α-1-receptors (α-1a, α-1b and α-1d subtypes) activates the Gαq subunit of G proteins and, therefore, Phospholipase C is activated. In contrast, the α-2-receptors (α-2a, α-2b, and α-2c subtypes) are coupled to Gαi/o subunit and, therefore, inactivate adenyl cyclase (AC). In contrast, the β-adrenergic-receptors family includes three subtypes: β-1, β-2, β-3, which activate the subunit Gαs and subsequently increase the intracellular cAMP levels. However, the β-2 adrenergic receptors may activate the Gαi and Gβγ subunits of G protein in some cases.
The dopaminergic receptors are clasified into two subfamilies. The D1-receptors (D1, D5) are coupled to Gαs proteins and their activation leads to estimulation of AC and subsequent increase in cAMP levels. On the contrary, the D2-receptors (D2, D3, D4) are coupled to Gαi proteins and their activation leads to inhibition of AC and decrease of cAMP levels. Members of the D1 and D2 receptors can form heterodimers that are coupled to Gαq proteins and their activation leads to stimulation of phospholipase C and intracellular calcium release.
Our preliminary studies showed the presence of adrenergic (both α and β types) and dopaminergic receptors (D1 and D2 subfamilies) in the heart tube (Figure 6) suggesting that catecholamines have the possibility to act through them. Further studies are needed to confirm the role of the specific receptor subtypes and signaling pathways in cardiogenesis.
4. L-DOPA and Dopamine induce cardiac differentiation
Differentiation of contracting cardiomyocyte requires the action of a combination of transcription factors, including Nkx2.5 and Tbx5 (17). The pattern of th expression suggested that catecholamines may play a role in cardiac development. Implanting microbeads soaked in L-DOPA and dopamine, laterally to one of the bilateral heart fields in cultured chick embryos, we could show that both L-DOPA and dopamine induce cardiac muscle differentiation. Cardiac transcription factors Nkx2.5 and Tbx5 were expressed in the ectopic tissue generated near the microbead (Figure 7A). Additionally, the sarcomeric protein AMHC1 (atrial myosin heavy chain), marker of terminal cardiac differentiation, was also induced, as reflected by in situ hybridization and immunostaining with MF20 antibody (Figure 7A). Moreover, the cells of the induced ectopic tissue developed myofibrils organized into sarcomeres, similar to those found in the cardiomyocytes of the primitive heart tube (Figure 7B). The link of TH to cardiac differentiation programs was further confirmed by blocking the endogenous synthesis of L-DOPA and dopamine, which led to a decrease in expression of AMHC1 (13).
Overexpression and knock-down of TH disrupts cardiac markers gene expression and affects chamber formation
We set out gain-of-function experiments using constructs that led to overexpression of TH together with GFP (Figure 4), or just GFP as a control, in electroporated chick embryos. One of the first features of anterior-posterior patterning is the restriction of the ventricular (VMHC-1) and atrial (AMHC-1) myosin heavy chain to the anterior and posterior pole, respectively, of the primitive heart tube (18-20).
We showed that TH overexpression increased and expanded the expression of the posteriorly restricted genes AMHC-1 and Tbx5 toward more rostral regions (Figure 8). These results suggested that TH is involved in the heart tube patterning by specifying the atrial region. In addition, TH also had major functional consequences, since the electroporated chick embryos displayed slower (53 vs. 100 beats per minute) and arrhythmic heart beats compared to controls (13). The use of morpholino-oligonucleotides against th mRNA allowed the knock-down of its expression in the chick embryo, leading to a decrease in AMHC1 and Tbx5 expression together with an atrophic sinoatrial region and oversized ventricular region (13). Thus, TH action not only induces cardiac differentiation in vivo but the catecholamine pathway regulates heart patterning, conferring atriogenic identity.
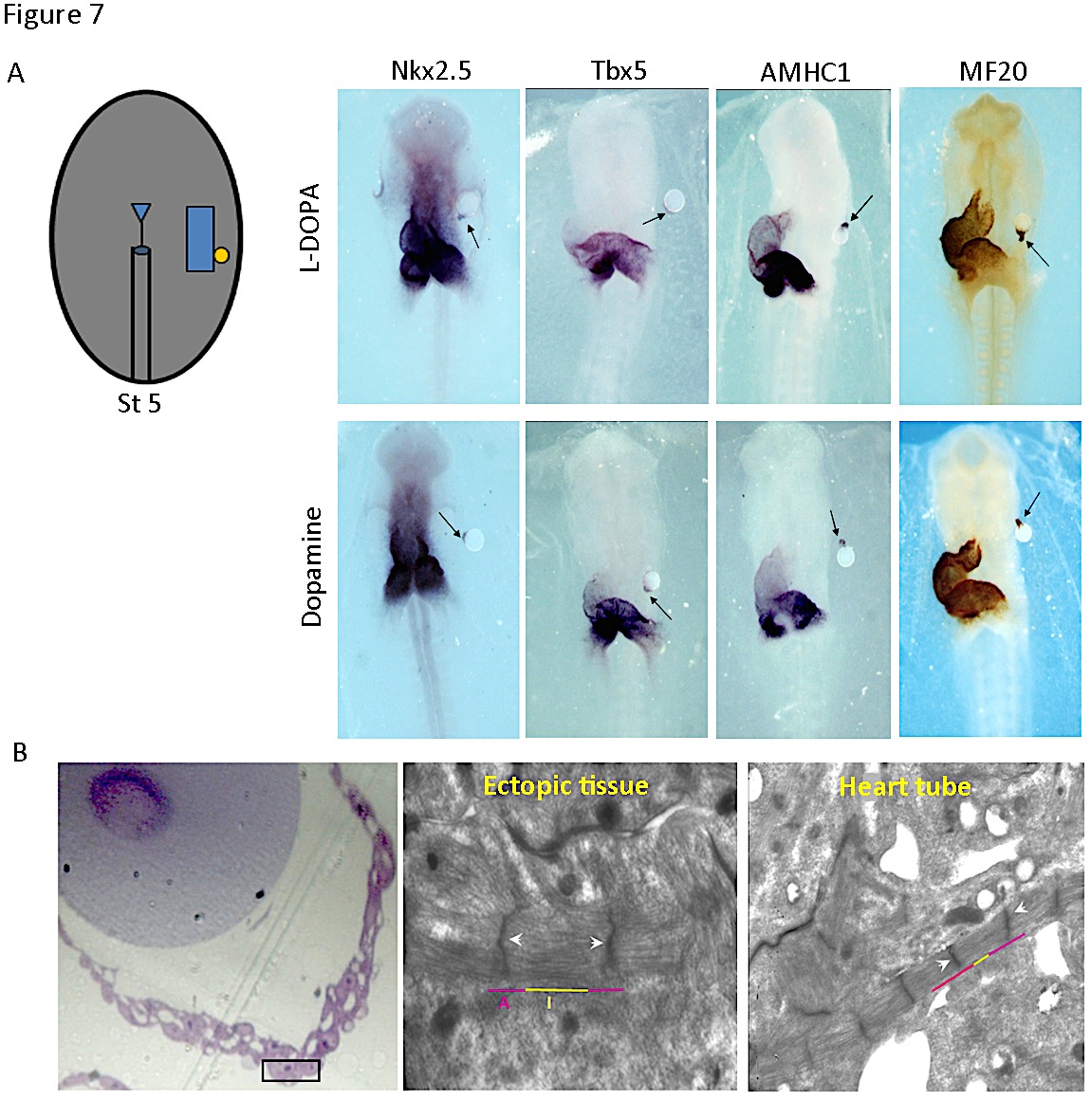
Figure 7. Induction of cardiac genes and cardiomyocytes differentiation by L-DOPA and Dopamine. A) Drawing corresponds to a stage 5 embryo scheme with a microbead implanted. Beads were soaked with either PBS (vehicle), or a solution of 10 Ķmol/L L-DOPA or dopamine. Stage 10-12 chick embryos were subjected to whole-mount in situ hybridization for the genes indicated (Nkx2.5, Tbx5, AMHC1) or immunohistochemistry for MF20. Ectopic tissue adjacent to the bead coated with L-DOPA or dopamine (arrow) expressed all markers. PBS did not induce any signal (not shown). B) Ultrastructure of the ectopic tissue induced. The white arrowheads indicate the Z bands and the purple and yellow lines delineate I and A bands of the cardiomyocytes adjacent to the bead. (Modified from [13]).
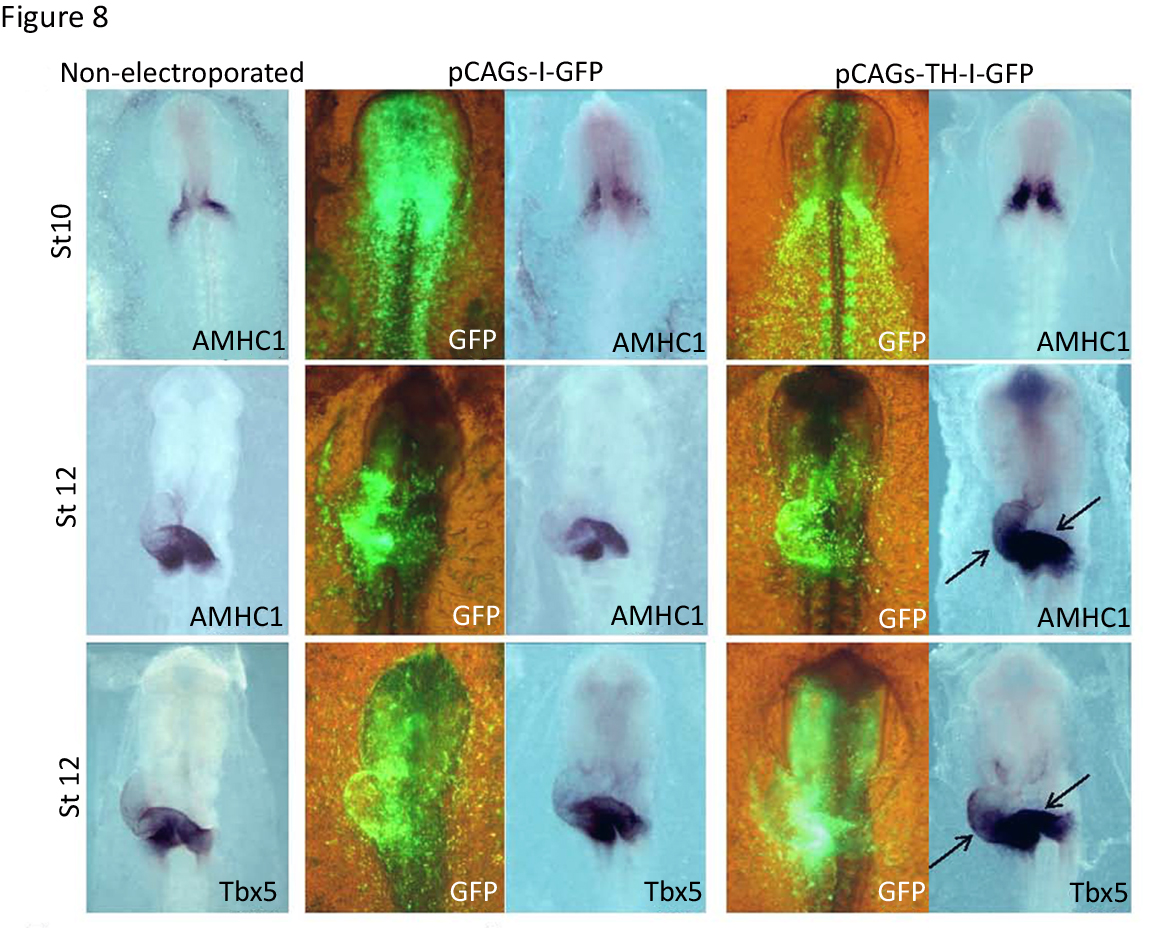
Figure 8. Effect of TH on anterior-posterior heart tube patterning and specification of chambers. Effect of TH gain-of-function on sino-atrial gene expression. Embryos from stages 10 or 12 underwent in situ hybridization for the indicated gene markers, AMHC1 and Tbx5. The control non-electroporated embryos are compared with embryos electroporated with either the control construct (pCAGs-I-GFP) or with the th expressing construct (pCAGs-TH-I-GFP). The anterior expanded expression of AMHC1 and Tbx5 in the heart tube is indicated by the black arrows. Visualization of GFP expression for the embryos electroporated is shown in the corresponding left panels. (Modified from [13]).
It is worth mentioning that the th global knockout mice, generated in 1995 by the group of Dr. Palmiter (21), is embryonic lethal starting at day E11.5-12.5. Although, the null mice die from apparent heart failure, a detailed characterization of the mouse embryo phenotype in organogenesis has not been carried out yet. This type of study would be required to unravel the role of catecholamines in mouse cardiogenesis.
5. Conclusions and future directions
The morfological and functional diversity of animal organisms depends on multiple mechanisms of gene regulation, especific in time and space, which permit the functional utilization of related transcripts (and their protein products) in distinct contexts along the life cycle. We have characterized the role of TH in cardiac development, focusing in the primitive heart tube formation. In the future we will try to define the mechanism of dopamine action and the receptors involved in cardiomyocyte differentiation.
It is intriguing as well the possible role of TH in the proepicardium, a transient structure in which we have found expression in later stages of cardiac organogenesis. In addition, TH is expressed in the mouse pancreatic primordium since its formation. We are now characterizing the expression pattern of TH during pancreas development, and the likely participation of catecholamines in differentiation programs leading to pancreatic endocrine cells. The field of the action of catecholamines in non-neural tissues development has turned out to be much broader than anticipated.
Acknowledgements
We thank present and past members of the laboratory, especially Enrique J. de la Rosa and Teresa Suárez, for their contribution to scientific discussions, the background data and specific figures modified for this article. This research was funded by grants BFU 2007-61055 and BFU 2010-15868 (MINECO) to F. de Pablo. PV is supported by a JAE-DOC contract from the CSIC.
references
(1) Harvey S. Extrapituitary growth hormone. Endocrine 38: 335-359 (2010).
(2) Hernandez-Sanchez C; Lopez-Carranza A; Alarcon C; de La Rosa EJ, de Pablo F. Autocrine/paracrine role of insulin-related growth factors in neurogenesis: local expression and effects on cell proliferation and differentiation in retina. Proceedings of the National Academy of Sciences of the United States of America 92: 9834-9838 (1995).
(3) de Pablo F; Roth J. Endocrinization of the early embryo: an emerging role for hormones and hormone-like factors. Trends in biochemical sciences 15: 339-342 (1990).
(4) Hernandez-Sanchez C; Mansilla A; de la Rosa EJ; de Pablo F. Proinsulin in development: New roles for an ancient prohormone. Diabetologia 49: 1142-1150 (2006).
(5) de la Rosa EJ; de Pablo F. Proinsulin: from hormonal precursor to neuroprotective factor. Frontiers in molecular neuroscience 4: 20 (2011).
(6) Martinez-Campos E; Hernandez-SanMiguel E; Lopez-Sanchez C; De Pablo F, Hernandez-Sanchez C. Alternative splicing variants of proinsulin mRNA and the effects of excess proinsulin on cardiac morphogenesis. FEBS letters 587: 2272-2277 (2013).
(7) Pendleton RG; Rasheed A; Roychowdhury R; Hillman R. A new role for catecholamines: ontogenesis. Trends in pharmacological sciences 19: 248-251 (1998).
(8) Hernandez-Sanchez C; Bartulos O; Valenciano AI; Mansilla A; de Pablo F. The regulated expression of chimeric tyrosine hydroxylase-insulin transcripts during early development. Nucleic acids research 34: 3455-3464 (2006).
(9) Akiva P; Toporik A; Edelheit S; et al. Transcription-mediated gene fusion in the human genome. Genome research 16: 30-36 (2006).
(10) Kim N; Kim P; Nam S; Shin S; Lee S. ChimerDB--a knowledgebase for fusion sequences. Nucleic acids research 34: D21-24 (2006).
(11) Parra G; Reymond A; Dabbouseh N; et al. Tandem chimerism as a means to increase protein complexity in the human genome. Genome research 16: 37-44 (2006).
(12) Buckingham M; Meilhac S; Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nature reviews 6: 826-835 (2005).
(13) Lopez-Sanchez C; Bartulos O; Martinez-Campos E; et al. Tyrosine hydroxylase is expressed during early heart development and is required for cardiac chamber formation. Cardiovascular research 88: 111-120 (2010).
(14) Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Developmental biology 258: 1-19 (2003).
(15) Chapman SC; Schubert FR; Schoenwolf GC; Lumsden A. Anterior identity is established in chick epiblast by hypoblast and anterior definitive endoderm. Development (Cambridge, England) 130: 5091-5101 (2003).
(16) Hamburger V; Hamilton HL. A series of normal stages in the development of the chick embryo. Journal of Morphology 88: 43 (1951).
(17) Takeuchi JK,;Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature 459: 708-711 (2009).
(18) Oana S; Machida S; Hiratsuka E; et al. The complete sequence and expression patterns of the atrial myosin heavy chain in the developing chick. Biology of the cell / under the auspices of the European Cell Biology Organization 90: 605-613 (1998).
(19) Somi S; Klein AT; Houweling AC; et al. Atrial and ventricular myosin heavy-chain expression in the developing chicken heart: strengths and limitations of non-radioactive in situ hybridization. J Histochem Cytochem 54: 649-664(2006).
(20) Yutzey KE; Rhee JT; Bader D. Expression of the atrial-specific myosin heavy chain AMHC1 and the establishment of anteroposterior polarity in the developing chicken heart. Development (Cambridge, England) 120: 871-883 (1994).
(21) Zhou QY; Quaife CJ; Palmiter RD. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature 374: 640-643 (1995).